(800) 958-8121
answers@mcguff.ca
English
2 Year Shelf Life
Extended shelf life for up to two years
Non-Corn
No detectable levels of allergens (amino acids, proteins, and polypeptides) including corn allergens.
Non-GMO
No genetically modified organisms.
Featured products
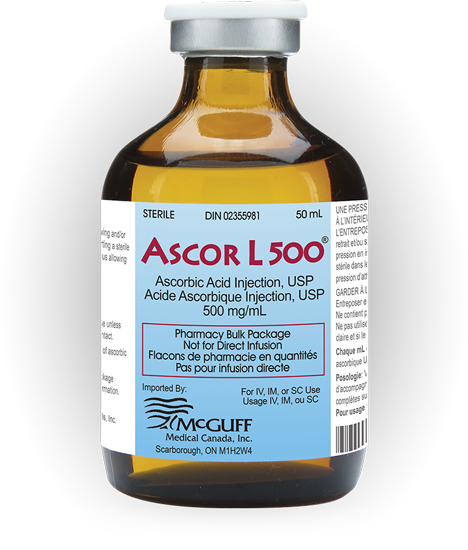
Ascor L 500® Ascorbic Acid Injection, USP (Vitamin C Injection)
McGuff Pharmaceutical's Ascor® consists of a 50mL pharmacy bulk package of sterile, preservative-free, pyrogen-free Ascorbic Acid, USP (500 mg/mL) in a solution containing Edetate Disodium (0.025%), Water for Injection (q.s.) and Sodium Bicarbonate for pH adjustment (pH 5.6 to 6.6).
$7,356.80